Czesc (hello in Polish) Trust you are well. I make no apology to print this long medical information. For it is by scaring parents that the so called elite crop the population, increase medical bills and make Bog Pharma more money, it is a cash cow. Then WHO issue another scary epidemic virus, which has been manufactures by some laboratory somewhere, and remember dead people do not make money for Big P , sick do.
Keep it from the parents: what the government doesn’t tell you about vaccinations
Government agencies are withholding worrying safety information about vaccinations, as secret minutes to key decision-making committee meetings revealGovernment health officials have for 30 years consistently misled parents about the dangers of the childhood vaccination programme by hiding or ignoring studies that show how vaccines like the measles–mumps–rubella (MMR) vaccine could cause permanent injury and even kill.
According to private minutes to meetings of the UK government’s Joint Committee on Vaccination and Immunisation (JCVI), members were made aware of a possible link between the MMR vaccine and autism 10 years before Andrew Wakefield published the controversial paper that eventually resulted in his being struck off the medical register.
From 1983 up to just recently, the minutes—obtained under Freedom of Information legislation—suggest that important information about vaccine safety that would almost inevitably have affected the take-up rate needed to achieve ‘herd immunity’ was not revealed to the public.
The JCVI acts as an advisory service to the UK’s health minister, and many of its committee members over the years have had ties to the pharmaceutical companies making vaccinations (see below).
Spin, not science
In an analysis of the private minutes covering monthly meetings of the JCVI between 1983 and 2010—which still contained many redactions and omissions—medical researcher Dr Lucija Tomljenovic from the University of British Columbia, Canada, makes eight serious charges against the committee and the UK’s Department of Health.
Charge 1. Instead of re-examining vaccine policies when new safety concerns were raised, the JCVI took no action, or skewed or selectively removed worrying safety data from reports released to the public, or made extensive efforts to reassure the public about the ultimate safety of the vaccines.
As long ago as 1981, the JCVI had extensive information concerning serious adverse reactions to the MMR vaccine, including sudden death and encephalitis, from studies going back to 1970. The JCVI said the high numbers of deaths and injuries were “surprising”.
After receiving even more worrying data on adverse reactions, a “commercial in confidence” JCVI subcommittee in 1986 decided to put a positive spin on the safety of two measles vaccines by reporting that “results showed that 70 per cent of children were well after receiving Attenuvax and 61 per cent after receiving Rimevax . . . [and] if children with mild general reactions were added to those who were apparently well, then the numbers associated with Attenuvax were 85 per cent and those with Rimevax 80 per cent.”
Another way of interpreting the same data is that between 15 and 20 per cent of children given one of the vaccines would suffer a serious adverse reaction.
The subcommittee agreed to stop the study, and concluded that the vast majority of adverse reactions were probably nothing to do with the vaccines but were, in fact, “temper tantrums”.
The same subcommittee was also told of 90 serious adverse reactions to the DTP (diphtheria–tetanus–pertussis, or whooping cough) vaccine, including convulsions, abnormal fever and two cot deaths.
By 1988 the JCVI was becoming concerned by an association in the public’s mind between vaccines and death or brain damage. Legal claims began to be brought to the courts, and JCVI members were given secret documents concerning vaccine safety levels on the express understanding that they were never to be made public. One paper on the scientific evidence of harm caused by the DTP vaccine conflicted with the current legal opinion that there was insufficient evidence to demonstrate that the vaccine caused permanent harm, the committee was told—in other words, there was sufficient evidence, but the government was sitting on it.
The following year the committee was given evidence from the National Institute for Biological Standards and Control (NIBSC) that indicated a link between the mumps component (Urabe Am9 strain) of the MMR vaccine and meningitis and encephalitis, an association that would be drawn 10 years later by Andrew Wakefield in his controversial Lancet paper. The NIBSC suspicions were vindicated by 1990 when 10 definite cases of meningitis/encephalitis were established by analyzing cerebrospinal fluid taken from recently vaccinated children.
Despite these findings the JCVI agreed to not make any changes to the then current vaccination policy although, by 1991, reports on adverse reactions from other countries, such as Canada and Japan, were mounting, prompting the committee to seek the guidance of the vaccine manufacturers. The drug companies concurred that the UK government’s approach of “surveying adverse events” was the correct one. But by 1992, SmithKlineBeecham, as the company was then known, decided to stop manufacturing the Urabe MMR vaccine on the advice of lawyers, and the JCVI was told that, in light of that sudden decision, it needed to “act quickly”.
In secret meetings with their European counterparts in September of that year, UK health officials decided not to revoke SKB’s licence to produce the vaccine because it would have “caused a worldwide vaccine crisis”.
Despite these growing concerns, the JCVI set aside £800,000 for a publicity campaign and £1.4 million to help meet increased vaccine costs, education and the “reprogramming of children’s computers”.
Charge 2. Health officials reduced the number of contraindications—the circumstances in which a vaccine should not be given—just to increase vaccination take-up rates.
By 1980, public confidence in the whooping cough vaccine (the pertussis element of the triple DTP vaccine) had reached a low point, and only 40 per cent of eligible children were being vaccinated. A new subcommittee was then created by the JCVI to address the problem; it was tasked with relaxing the parameters of defining the ‘at-risk’ children. The subcommittee had been told that “the right balance had to be struck between the need to keep acceptance rates for vaccination as high as possible and the need to protect groups of children who had an increased risk of adverse reaction to vaccination” (the contraindicated vaccine recipients).
But the emphasis was on the reputation and safety profile of the vaccination and not the wellbeing of the vaccinated child. Respiratory disease was one contraindication that should be removed, some committee members mooted, while others pointed out that the contraindication “protected the reputation” of the vaccine. Respiratory disease was often associated with sudden infant death syndrome (SIDS) and the vaccine could be dragged into the controversy if those children received the vaccine.
A similar argument was put forward to members who wanted epilepsy to be removed as a contraindication. Again the vaccine could be blamed if the child suffered convulsions or seizures. Yet even in healthy children, the vaccine was causing fits, and the child stopped developing. “It is very difficult to assess this as a random event,” the committee was told.
However, to fulfil its brief the subcommittee finally agreed that there should be “special consideration”, but not necessarily exemption, of children with cerebral damage from birth, with a history of convulsions, with a family history of epilepsy, with developmental delay or with neurological disease. The risk posed by the vaccine in the child with a familial history of epilepsy was “slight”, said the subcommittee, while developmental delays and neurological disease were stable conditions and so would not be worsened by the vaccine.
These statements, designed to reassure parents, had little or no basis in fact and were not supported by any evidence.
Although the subcommittee was keen to play down the risks of the DTP vaccine, it had been told of 95 adverse reactions, including one death, in children given the vaccine during the four months leading up to September 1986. The report concluded that “there is reason to believe that the increased relative risk of prolonged convulsions after DTP was a real one”.
Not only was it real, the risk was also high, the subcommittee was told after reviewing 12 cases of vaccine-induced encephalopathy, which included two deaths and five cases of long-term impairment.
Despite having these data, the subcommittee issued reassuring statements to parents that downplayed the risks—while also advising the vaccine manufacturers to alter their data sheets to include the risks and so avoid legal action later.
Charge 3. The JCVI worked closely with the vaccine manufacturers to amend the accompanying data sheets even though they often conflicted with its official advice to parents.
From 1987 onwards the JCVI worked closely with the pharmaceutical company Mérieux, which was making a booster MMR vaccine and a DTP vaccine, to encourage it to change the data sheets so that they were in line with forthcoming changes to the Department of Health’s own guidelines to doctors. Without that conformity, the JCVI was worried that the UK government would be leaving itself open to damages claims.
But the drug company felt it could not make the changes while litigation, brought by the parents of a child injured after being given the pertussis vaccine, was ongoing. Nonetheless, the JCVI had private meetings with the Association of the British Pharmaceutical Industry and solicitors for the Department of Health to see if some help could be given to Mérieux. The discussions were described as “commercial” and “in confidence”.
Presenting a united front became imperative after the JCVI received a report that year from the Committee on Safety of Medicines (CSM), which stated: “No scientifically unassailable link has been established between DTP immunisation and serious neurological illness, but we have come to the conclusion, on the basis of all present evidence, that there is a prima facie case that such a link may exist. We would also agree that the evidence suggests that the vaccine causes convulsions in some children.”
Despite its concerns, the CSM supported the JCVI’s view that the DTP vaccine was safe. According to Dr Tomljenovic, the JCVI made legal “discovery” of negative reports difficult and so evaded potential legal repercussions.
Charge 4. The JCVI persistently relied on dubious studies, and dismissed independent research, to promote vaccine policies.
To promote the MMR as a safe vaccine, the JCVI always welcomed studies that appeared to support that view while dismissing any that raised concerns. It greeted one population-based study, which could find no link between autism and MMR, as “persuasive” even though it is not the purpose of epidemiological studies such as this to look for causation.1Indeed, four years later the prestigious Cochrane researchers looked at the same study and concluded that “the number and possible impact of biases in this study were so high that interpretation of the results is impossible”.
In the same Cochrane review, which looked at 31 studies of MMR and autism and Crohn’s disease, the researchers concluded that the studies were so poorly designed that they were “largely inadequate”.2
The JCVI reserved a similar critical eye only for those studies that suggested problems with the MMR. In 2002 its experts reviewed five critical studies, including one that found a direct link between the MMR and autism, and concluded there was “no evidence” of any causation (see below).
Charge 5. The JCVI persistently played down safety concerns while exaggerating vaccine benefits.
The number of legal claims over injury from the pertussis vaccine was reaching a crescendo by 1985, and the JCVI wanted to put a lid on rising public concerns. In one confidential paper issued in 1986, it said it “deprecated” the use of the term ‘brain damage’ when talking about the vaccine as “the public might consider [it] a permanent entity”.
The JCVI clearly didn’t want to find out if any damage was permanent as, in the same paper, it stated that it was “unreasonable to ask paediatricians to report for a period of six years”. This was a strange decision as one review, known as the Meade Panel Study, reported to the JCVI examples of children “who had a fit soon after vaccination which was followed by a fit at a later time and then followed by cessation of development”. The length of time that development stopped was not known.
The vaccine also triggered encephalopathy, a disease that damaged brain function, the JCVI was told in a confidential paper, and one-third of these cases resulted in a permanent handicap for at least a year following vaccination.
The JCVI was also told that the DTP vaccine could cause febrile convulsions lasting more than 10 minutes or repeating over a 24-hour period, and that 10 per cent of these could cause “permanent handicap”.
Despite these clear descriptions of permanent harm, the JCVI thought it best to keep the facts from the public. The minutes of one meeting recorded that “if the public was given a risk ratio—any ratio—they would see it as a scientifically proven risk. It was therefore preferable not to use insecure figures if possible but to stress the benefits from vaccination.”
Charge 6. The JCVI promoted new vaccines of questionable efficacy and safety into the routine vaccination schedule on the assumption that licenses would eventually be granted.
In May 1999 the JCVI met to discuss the introduction of new group C meningococcal vaccines designed to protect against septicaemia and meningitis. Three brands of the vaccine were likely to be adopted as part of the mass vaccination programme, and members were reminded that the issue was “extremely sensitive, commercially and politically”. Because of the sensitive commercial nature of the discussions, four of the committee members declared a conflict of interest as they had close ties to the drug companies manufacturing the vaccines, but rather than barring these members, the committee allowed them to stay as “they would be able to provide a valuable input”.
The Department of Health and the JCVI seemed determined to introduce the new vaccine as quickly as possible, although there was no evidence that it was effective. “To actually test the efficacy . . . it would be necessary to introduce the vaccine and then conduct a Phase III or Phase IV study to test efficacy; this would be very difficult to do and would delay introduction by three to five years,” the JCVI was told.
Moving on, the JCVI then had to decide on the age group that should be given the vaccine. One committee member said “there was very little to choose between the priority age groups” and suggested that infants were easier to target. It was agreed that all infants should receive the vaccine, even though nobody knew if it would work.
Within a year the first safety worries surfaced. Headache and muscle stiffness were common side-effects, although the symptoms were not the result of meningitis since, wrote the JCVI, “the vaccine could not cause this”. The committee also noted that “this information would not have been available without the cooperation of the manufacturers. This had given everyone much more confidence in the vaccine programme and was a unique cooperation.” The statement suggests this was the first time ever that the drug companies had shared adverse events information with the UK officials.
But confidence was beginning to wane by October 2000, when 14 deaths were linked to the vaccine, including seven SIDS cases and two because of meningitis. Again the JCVI moved to put a lid on public concerns. It felt the statement from the Medicines Control Agency that there was “no evidence that the vaccine caused meningitis” was too mild and instead proposed the statement that “the vaccine categorically did not cause meningitis”, although it had no evidence to support that claim.
In fact the JCVI was told there had been 17,000 adverse events reported by family doctors using the ‘yellow card’ system for reporting adverse reactions, which represented one adverse event per 2,000 doses of the new vaccine. Department of Health solicitors also told the JCVI that the yellow card system was not routinely used by doctors and so the true extent of adverse reactions was probably far higher than that reported.
Charge 7. The JCVI actively discouraged research into vaccine safety issues.
As early as 1985 the numbers of infants who had died immediately after vaccination were beginning to cause concern, and an expert from the London School of Hygiene and Tropical Medicine was asked to review the data. In a letter to the JCVI, he thought that the government’s estimates of vaccine-related SIDS were about right—suggesting some causal link, albeit small—but “given the importance of the subject, a more thorough examination of the subject seems appropriate”.
But scientists from Nottingham University, who were sent the letter, strongly advised against further investigation. “There is no foolproof method of discrediting the hypothesis [that vaccines cause SIDS]”, they wrote, suggesting that they had already made their minds up, and they advised that nobody should look too closely at the issue.
This reluctance in the scientific community was highlighted by the European Medicines Agency’s Professor Luigi Matturri, who noted that full post-mortem examinations—including brain-stem analysis—were not carried out on five infants in Germany who had died within 24 hours of vaccination. This prompted a letter from two researchers who asked: “The main problem is that vaccine specialists have failed for decades to establish any tests or other criteria to find out if adverse events are linked to vaccinations or not. To our knowledge they did not even try hard—why?”3
Underpinning this reluctance are apparently two assumptions: that vaccines are inherently a ‘good thing’, so any suggestion that they could cause harm would reduce the numbers having them, thus impacting on the protective effects of ‘herd immunity’; and that vaccines are assumed to be safe. This is perhaps why no one has looked too hard for serious adverse effects; as America’s Food and Drug Administration has stated, “Historically, the safety assessment of preventive vaccines has often not included toxicity studies in animal models. This is because vaccines have not been viewed as inherently toxic”.4
Charge 8. The JCVI deliberately took advantage of parents’ trust in vaccinations to promote a scientifically unsupported immunization programme that could put certain children at risk of severe long-term neurological damage.
In October 2010 the JCVI decided to make fundamental changes to the UK’s immunization programme by introducing a six-in-one jab in one visit rather than spreading them out over a few months. At 12 months an infant would receive vaccinations for Haemophilus influenzae and meningitis C, measles, mumps and rubella, and pneumococcal infection, all in one day.
The Department of Health said this simplified policy would streamline the process, while “independent scientific research has shown that providing these vaccines at the same time is safe, effective and more convenient for parents”.
The statement isn’t true. The ‘independent’ research was in fact carried out by the Department of Health itself, while the safety of the vaccines was assessed at seven days after vaccination and then only for localized reactions such as swelling and tenderness immediately around the site of injection.
In 2009 the JCVI had started the ball rolling by concluding that there is “no scientific reason to keep the . . . vaccines separate”. Again, the statement is untrue. A year earlier the JCVI counterpart in the US, the Advisory Committee on Immunization Practices (ACIP), had recommended dropping a four-in-one jab, which combined the MMR with one for varicella (chickenpox), after it was found to double the risk of febrile seizures compared with the MMR vaccine on its own. In the same year the European Medicines Agency had recommended the withdrawal of a six-in-one (hexavalent) vaccine for safety reasons.
Ignoring these warnings from other countries, the Department of Health thought it was “unwise” to offer parents a choice between the current policy of spreading the vaccines over several months or giving their child the six-in-one. Instead, health professionals should reassure parents that the new strategy was “entirely safe” and that, partly in response to the Wakefield controversy, the “MMR is safe”. Yet, just months earlier the UK government had paid out damages of £90,000 to the parents of Robert Fletcher, who had suffered epilepsy and severe mental retardation following the “entirely safe” MMR jab.
The JCVI also seems to have a short-term memory. In 1974 it was advised that “[as] an interval in the administration of live vaccines [is] advocated in view of the probability of adverse reactions . . . the committee agreed that it would be inopportune to change the guidance that an interval of at least three weeks should be allowed to elapse between the administration of any two live vaccines.”
In spite of this, the health professionals had to downplay any risks. The best strategy, the Department of Health advised in a circular to clinics, was to hand out a sheet listing possible adverse reactions to parents “immediately before vaccination so that parents feel they have been given advance warning, but do not dwell on the content to the extent that they begin to worry”.
The path to hell
These recommendations were based on market research carried out by the Department of Health among parents to determine how best to implement the new six-in-one vaccine and how to gauge resistance to the MMR vaccine in particular. What came out of this ‘attitudinal’ research was the vast gap between the trust of parents and the cynical fast-and-loose approach of policy makers.
Typical comments from parents revealed an assumption that vaccines have been thoroughly tested and that policy makers have the best interests of their children at heart.
In a sense the latter statement is true: vaccine policy makers truly believe that vaccinations can save countless lives, although this protective effect can be achieved only through ‘herd immunity’, when around 95 per cent of the targeted population has been immunized.
It’s a belief that surpasses science and takes on more of a religious zeal so that any inconvenient truths—such as adverse reactions up to and including death—are discounted or disbelieved. They just don’t fit with the paradigm that vaccines are safe and effective.
Parents don’t want religious zeal or the preservation of paradigms. They want to know their children’s safety is paramount—and yet it isn’t. Vital safety information is being deliberately withheld, which makes the parents’ consent to have their children vaccinated not informed—raising the possibility that every vaccination is an illegal act.
References
1. Pediatrics, 2001; 108: e58
2. Cochrane Database Syst Rev, 2005; 4: CD004407; 2012; 2: CD004407
3. Vaccine, 2006; 24: 5781–2; author’s reply 5785–6
4. US DHHS, FDA, Office of Women’s Health. Workshop on: Non-clinical safety evaluation of preventive vaccines: Recent advances and regulatory considerations, volume 1. Washington, DC: Miller Reporting Co, 2002
THE MMR AND AUTISM
Andrew Wakefield lost his job as a consultant at the Royal Free Hospital in London, and eventually his licence to practise medicine, after he suggested a possible link between the MMR vaccine and autism. He was found guilty of fraud in preparing the 1998 paper in which he first postulated such an association.
The General Medical Council hearing that struck Wakefield off the medical register in 2010 had been told that no researchers were able to replicate his original findings of a possible connection between a gut disorder caused by the measles component of the MMR vaccine and autism.1
The JCVI jumped on this statement as further evidence of the safety of the vaccine—but, yet again, it wasn’t true. In 2002, researchers from Utah State University analyzed blood samples from 125 autistic children and compared them with blood taken from 92 healthy children. In 75 instances in the autism group, antibodies in the blood indicated an abnormal reaction to the vaccine. The antibodies attack the brain by targeting the building blocks of myelin, the insulating sheath that covers and protects nerve fibres. As a result, the nerves fail to develop properly and so possibly affect normal brain functioning. None of the non-autistic children had such antibodies in their blood samples.
Lead researcher Dr Vijendra Singh, an immunologist, said there was a relationship between the abnormal reaction to the vaccine, which happens in some children, and autism.2
References
1. Lancet, 1998; 351: 637–41
2. J Biomed Sci, 2002; 9: 359–64
Herd, but not seen
Vaccines protect the general population when 95 per cent or more of the targeted at-risk group is vaccinated, according to the concept of ‘herd immunity’. This one theory can explain the JCVI’s consistent blocking and denial of evidence of adverse reactions, as any ‘bad news’ like this could affect the take-up rate.
But does the theory hold up? According to the evidence, it doesn’t. After one outbreak of measles in Corpus Christi, Texas, in 1985, researchers discovered that 99 per cent of the children affected had received the measles vaccination and at least 95 per cent were supposedly ‘immune’, according to their blood samples.1
Three years later, there was an outbreak of 84 measles cases at a college in Colorado, and yet 98 per cent of the students had been vaccinated and were still immune, according to blood-serum analyses.2
This was followed by a chickenpox outbreak at a school where 97 per cent of children had been vaccinated. Those who had been vaccinated more than five years previously were especially at risk, the researchers concluded.3
References
1. N Engl J Med, 1987; 316: 771–4
2. Am J Public Health, 1991; 81: 360–4
3. Pediatrics, 2004; 113: 455–9
Keeping up standards
As with anyone holding public office, the members of the UK’s Joint Committee on Vaccination and Immunisation (JCVI) are supposed to meet exacting standards. These are encapsulated in the ‘Seven Principles of Public Life’, set out by the Nolan committee, which include:
- Selflessness. Holders of public office should take decisions solely in terms of the public interest. They should not do so in order to gain financial or other material benefits for themselves, their family or their friends.
- Objectivity. In carrying out public business, holders of public office should make choices on merit.
- Accountability. Holders of public office are accountable for their decisions and actions to the public and must submit themselves to whatever scrutiny is appropriate to their office.
After reading the secret minutes reported in this article, how many of these principles do you consider JCVI members have broken?
- Openness. Holders of public office should be as open as possible about all the decisions and actions that they take. They should give reasons for their decisions and restrict information only when the wider public interest clearly demands it.
Turning to the JCVI’s own rules about personal financial interest, “if a member has in the last 12 months received, or plans to receive, a financial payment or other benefit from a business or representative body relating to vaccines or any other product or service, including carrying out consultancy or fee-paid work, the member must declare an interest . . . [I]f this interest is specific to an agenda item and the payment or other benefit is connected specifically with the product under consideration, the member will be required to absent him/herself from the discussion and any subsequent vote”.
Yet according to the minutes regarding the introduction of the group C meningococcal vaccine, members with a direct link to the manufacturers were allowed to stay as “they would be able to provide a valuable input”.
How many other times has this rule been broken?
Bryan Hubbard
WDDTY vol 23 no 12
http://wakeup-world.com/2013/04/03/breaking-air-force-chemtrails-manual-available-for-download/
http://wakeup-world.com/2013/04/02/fluoride-the-deadly-elixir-in-our-water-supplies/
I appreciate I must be boring the pants off of you, as I keep on about fluoride, but from my knowledge in Forensics and talking to water plant scientists who are willingly privately to share on this dangerous, deadly toxic poison.
Just a sample quote from above:
Poison Warning
All US toothpaste tubes have a poison warning on them by Federal law. Our tube says; keep out of the reach of children… supervise brushing… don’t swallow… use no more than a pea sized amount. The US tube continues on with; IF YOU ACCIDENTALLY SWALLOW MORE THAN THE PEA SIZED AMOUNT, CONTACT A POISON CONTROL CENTER IMMEDIATELY! The reason this warning appears is because there is enough fluoride in a tube of toothpaste to kill a child! The amount in the tube is 1/5th of a level teaspoon. Amazingly fluoride is a cumulative poison and is more toxic than lead. However, you have a choice whether you use it or not. Once it gets dumped in your drinking water all choice goes out the window.What they are proposing to dump in is not fluoride… it is hydrofluorosilicic acid. Which is the toxic waste of the phosphate fertilizer and aluminium and nuclear industries. It has uranium, strontium, lead, arsenic, mercury, aluminium, and 23 other contaminants, as well as toxic fluoride. It is a toxic cocktail being promoted as FLUORIDE. They get away with this because the government and industry are keeping you in the dark and getting you to pay for their toxic waste, which would cost them money to safely bury it.Europe and Fluoride
98% of Europe has banned or rejected water fluoridation. Why, don`t they want good teeth? A lot of European countries have better teeth than the Republic of Ireland, who have been fluoridated for 45 years! What is worse, Spain and Holland have a fluoride toxic dumping problem caused by their industries and their solution is to ship it to Britain and Ireland and get you to eat it! Holland stopped adding fluoride after a group of doctors carried out a double blind study proving that fluoride caused osteoporosis and arthritic pains, IBS, thyroid dysfunction, Skin rashes and dental damage called Dental Fluorosis.. After these doctors ended fluoridation, they fought on and got the Constitution rewritten to prevent fluoride from ever being added again!

Whenever things get 'sticky' in the UK politically and financially, fluoride is introduced. It dumb's down resistance and uprising, the UK takes toxic waste which no one will bury or dump, like a out of control greedy psychopath anything for money and a fix.
UK is contemplating using fluoride in drinking water. When I did my lectures and workshops at the EU in Brussels, many of the non British MEP'S (representatives) would openly say to me 'do you know what a whore is? I would say 'of course' their reply, 'nearly every MEP thinks that Britain is the whore of Europe, they would kindly deprive and kill their own people, rob by taxation and lies, but most of all take the shit of Europe and dump it in your ground and at the same time protesting innocence,diplomacy and tact, fairness and your upper class a bunch of rich hedonistic perverts'. Unfortunately when we see the 'legal cover ups and banks conning the people, and all the stiff British upper lip' it is akin to the USA claiming to be the land of the free.
When I was in forensics we went into many a brothel to process some muggings, rape, murder, robbery and I found the girls 'off duty' so to speak refreshingly honest in a macabre sort of way, some of them were hard working and their situation was such that this was the only thing to do, some were forced into this by totally unscrupulous traffickers, those who were forced, or tortured they had no choice and that they should suffer and I felt helpless to help them in their plight, however, at least getting evidence would help prosecute these sharks, it disgusted me , I felt repulsed that that humans could do this to humans. Many of these girls from East Europe, the Philippines and elsewhere lived in cramped dirty conditions, and this could bring disease, many of the girls were forced into drugs and became junkies, another rape of the feminine energy, another assault on Mother Earth's daughters. They were who they were, they were not politicians, they wanted money for whatever they felt was there need, men knew the orgasms were in most cases fake, the punters knew this. Politicians fake their promises and lie like I never had heard before. Like pop stars they begin to believe their own rhetoric and spiel, they believe through repetition and rote their publicity and advertising. One of my nieces said to me when she was a teenager,'Uncle how do you know when a politician is lying' I said 'I have no idea' she replied 'when they open their mouths to speak' For me it about sums it up.
When I was in forensics we went into many a brothel to process some muggings, rape, murder, robbery and I found the girls 'off duty' so to speak refreshingly honest in a macabre sort of way, some of them were hard working and their situation was such that this was the only thing to do, some were forced into this by totally unscrupulous traffickers, those who were forced, or tortured they had no choice and that they should suffer and I felt helpless to help them in their plight, however, at least getting evidence would help prosecute these sharks, it disgusted me , I felt repulsed that that humans could do this to humans. Many of these girls from East Europe, the Philippines and elsewhere lived in cramped dirty conditions, and this could bring disease, many of the girls were forced into drugs and became junkies, another rape of the feminine energy, another assault on Mother Earth's daughters. They were who they were, they were not politicians, they wanted money for whatever they felt was there need, men knew the orgasms were in most cases fake, the punters knew this. Politicians fake their promises and lie like I never had heard before. Like pop stars they begin to believe their own rhetoric and spiel, they believe through repetition and rote their publicity and advertising. One of my nieces said to me when she was a teenager,'Uncle how do you know when a politician is lying' I said 'I have no idea' she replied 'when they open their mouths to speak' For me it about sums it up.
Street prostitute, the dangers of getting into a vehicle with a possible maniac, this happens a lot, and many girls have been raped and murdered or badly hurt.
As a therapist in central London, Piccadilly, after my work, I voluntarily worked as a therapist and had many a street and brothel lady arrive for help. Many lonely, emotionally cut off and this above shows to me the long tunnel of hope and despair.
This is a brothel in Melbourne, Australia from years back, before I was born, it depicts the 'oldest game on Earth'.Jesus had his feet washed by a 'lady of the night'. I labour this point because the feminine is being slaughtered, and by that not just females, but the feminine balance in men, and I do not mean gay men or women. Nature, innocence, compassion, altruism all being mercilessly exploited. By the way gay people have their balance and are like hetro's kind, caring, also angry, violent, etc. and like us all have the whole gamut of emotion and attributes. We are all human, what happens after indoctrination is another story.
Exotic metals and Magnets. A 10 min video of fascinating metals.
- Arthroscopic knee surgery for osteoarthritis and/or torn meniscus is one of the most unnecessary surgeries performed.
- Research has shown arthroscopic knee surgery works no better than placebo surgery, and when comparing treatments for knee pain, physical therapy was found to be just as effective as surgery, but at significantly reduced cost and risk
- Recent research also shows exercise is just as effective as surgery for people with a chronic pain in the front part of their knee, known as chronic patellofemoral syndrome (PFPS), which is also frequently treated with arthroscopic surgery
- In one recent study, 84 percent of patients reported significantly less pain and elbow tenderness at six months following treatment with platelet rich plasma (PRP), a component of whole blood that contains a number of growth factors that takes advantage of your body’s natural healing process
- Natural tips for pain relief and cartilage loss are listed, including: exercise, optimizing your vitamin D, K2, and sulfur levels. Natural pain relieving agents you can try include astaxanthin, omega-3, hyaluronic acid and curcumin, among others
- A new study yielded some surprising findings about the effects of exercise on the circadian rhythms of mice
- In this study, all of the exercising mice showed benefits to their circadian cycles—however, the time of day they exercised determined their degree of benefit; mice that exercised in the afternoon showed the greatest benefits, as opposed to those exercising in the morning or evening
- Circadian rhythms are your body’s internal clock, largely based on signals of light and darkness, which sends signals to all of your cells about when to conduct certain physiological processes
- When your circadian clock is disrupted, such as by sleeping in a room that isn’t dark or working the night shift, you have an increased risk for type 2 diabetes, obesity, memory loss, mood disorders, certain types of cancer, and a number of other serious health problems
- This UCLA study is an interesting contrast to other research showing that exercising in the morning before breakfast, in a fasting state, better lowers insulin resistance and optimizes how your body recycles proteins into muscle.
Published on 27 Mar 2013
Scientists at the University of California Los Angeles have found a way to create stunningly detailed 3D reconstructing of platinum nanoparticles at an atomic scale. These are being used to study tiny structural irregularities called dislocations.
Read the paper here: http://dx.doi.org/10.1038/nature12009
This short video depicts an unknown possible energy weapon used in Iraq. Listen to the surgeons who could not find bullets, shrunken bodies. Could this be a cattle mutilation link, when I interviewed certain whistle blowers in the late 80's they told me that cattle mutilations and abducted people by ET were often 'home grown' that were 'black ops' or in those days we called them the secret government, and they were experimenting on energy weaponry and human genome, along with the cloning humans with animals. See Post 20 and scroll down to transhumanism by Tom Horn.
The jury is out on this one.
Black dignitaries discuss the gun laws(3 mins)
The above 10 mins video is INCREDIBLE civil war in the USA, the body bags, FEMA, concentration camps. This is happening, is Europe next?Published on 27 Mar 2013Dr. Jim Garrow is a renowned author and whistleblower who has been nominated for a Nobel peace prize for his humanitarian work. He is the author of The Pink Pagoda: One Man's Quest to End Gendercide in China. He has spent over $25 million over the past sixteen years rescuing an estimated 40,000 baby Chinese girls from near-certain death under China's one-child-per-couple policy by facilitating international adoptions. He is the founder and executive director of the Bethune Institute's Pink Pagoda schools, private English-immersion schools for Chinese children. Today he runs 168 schools with nearly 6,300 employees. Dr Garrow was recently contacted by a high ranking military official who implored him to reveal the truth about a "litmus test" that is being proposed by the Obama administration to the military asking the question "will you shoot Americans if they won't give you their guns?" For more info on the work of Dr.Jim Garrow visit: http://pinkpagoda.org
Abby Martin talks to US Congressman, Dennis Kucinich, about the 10th anniversary of the Iraq war and other issues that set him aside from the average establishment politician. A brave and courageous man.
Your birth certificate maybe more than you think. In some cases you can look it up in the stock market.
n the 1970s and 1980s, a generous spirit suffused the internet, whose users were few and far between. But today, the net is ubiquitous, connecting billions of people, machines and essential pieces of infrastructure -- leaving us vulnerable to cyber-attack or meltdown. Internet pioneer Danny Hillis argues that the Internet wasn't designed for this kind of scale, and sounds a clarion call for us to develop a Plan B: a parallel system to fall back on should -- or when -- the Internet crashes. TEDTalks is a daily video podcast of the best talks and performances from the TED Conference, where the world's leading thinkers and doers give the talk of their lives in 18 minutes (or less). Look for talks on Technology, Entertainment and Design -- plus science, business, global issues, the arts and much more. Find closed captions and translated subtitles in many languages athttp://www.ted.com/translate
A lot of people I know are feeling pressurised, unable to meditate, restless a sort of a innate hurly burly, as if rushing to an unknown future, as things change so fast, as Danny Hillis said above, the internet has changed so fast, and even in the last hour.
Many years back in one of my studies for a MS.C I did a project and it was part of dissertation and I named 'The ice cubes in the Jar' there was a lot of stuff in thermodynamics, electron track change and so on, the simple version here is this:
It becomes obvious as each piece of ice interrelates, interfaces, with the one's around it, their relationships change as the ice melts. Eventually the ice melts and all is one in the water. The water becomes warmer, and then applied heat, causes it to boil, it boils to steam, and then back to source. The internet is like this, like a growing organic body, each new piece of information, changes the shape, body and relationship to billions already encapsulated to this body of information. So we see information is the energy of change.
One can now see that the concerned one world elite, a religion or cult, can be scarred of this 'new growing baby, adolescent this whatever growing being, and feel their 'bit' of their protected world is being gazumped' (Gazumping occurs when a seller (especially of property) accepts an oral offer of the asking price from one potential buyer, but then accepts a higher offer from someone else. It can also refer to the seller raising the asking price at the last minute, after previously orally agreeing to a lower one. In either case, the original buyer is left in the lurch, and either has to offer a higher price or lose the purchase.[1] The term gazumping is most commonly used in the UK and Australia, although similar practices can be found in some other jurisdictions).
I borrow the word gazump, which is a colloquialism as it seems apt and fits the above. Each piece of information melts a bit more ice, the interface changes, and yet there is a blockage as the challenge of this new piece threatens the old order, so there can be a real problem for the retainer of the old, shut down the net, block it and so on, this interacting I borrow the colloquialism gazump as it seems to fit so aptly. Each piece of this enormous input challenges and is fast growing and like my Inverted V, the tipping point, Einstein-Bose condensate, Prigogine dissipative structures, we will come to this point of saturation and then bust, become the caterpillar entering the chrysalis and then what? A new butterfly, maybe our brains will be like the binaural brain (see we post 10, scroll down to Steve Judd, Prigogine as well).
So we see this fast growing world of technology, no sooner a new lap top, tablet, or whatever, usually already a few years out of date, held back so as to sell the model, the upgrade. Our brains may as above in Steve Judd's quote and my synopsis, become the third brain, maybe we will be telepathic, there are some people with amazing powers to read really fast, and I mean really fast and retain the information and locate it accurately. Super fast reading, memory, and then maybe a wipe out as Patrick Geryl (Post 20) suggest is the form to break it down, the structure is polluted, worn out, the caterpillar fat and ready for change, the death call is there and clarion.
We may get to such a fast spin as in my stargate theory (Post 3 and 20)that we whirl away and create a 'mental' black hole, a wormhole, like a helter skelter, and find ourselves in a parallel world, another dimension and we realise our world was only a consensus of agreements, so agreed upon, that we all are brain washed and thought it was solid, real, reality, then we wake up as we are spun by the momentum of rapid change that we find ourselves in a space of evolutionary rapidity and in the vortex to another world, hopefully as Prigogine would say a higher and better form. This Tipping point, this apex of the V is being sensed and my feeling is that the energies of evolution are pushing for change. Who will survive the ride? It is the equation: 'For the amount of absorption and surrender to the New Way arriving, and to the least resistance to Universal Law, the Cosmic Order, Natures Way, and to the acceptance of the clarion call so will it be, so be it'
A powerful earthquake struck near Iran's Gulf port city of Bushehr, killing at least 30 people and injuring 800 but leaving Iran's only nuclear power plant intact, officials said.
Shocks from the quake were felt across the Gulf in Bahrain, Kuwait, Qatar and the United Arab Emirates, provoking panic and the brief evacuation of some office towers, residents and media said.
Bushehr provincial Governor Fereydoon Hasanvand told state television at least 30 people were killed while more than 800 people were hurt and receiving medical attention. 09-04-13
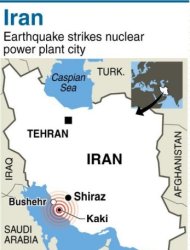
VIENNA (Reuters) - Iran has told the U.N.'s International Atomic Energy Agency that an earthquake that struck close to the country's only nuclear plant on Tuesday did not damage the facility, the IAEA said.The Vienna-based U.N. body said the quake - which Iranian media said killed 30 people as it devastated small villages - hit about 91 km (56 miles) from the Bushehr nuclear power plant."Iran has informed (the IAEA's Incident and Emergency Centre) of the event, reporting that there has been no damage to the Bushehr Nuclear Power Plant and no radioactive release from the installation," the U.N. agency said in a statement.Based on this information and the IAEA's own seismic analysis of the earthquake's magnitude, location and other factors, the agency "is not currently seeking additional information from Iran," the statement added. (extending condolences to those families of their loss and healing wishes to Iran and It's peoples).
The Hopi Elders Speak
We Are the Ones
We've Been Waiting ForYou have been telling the people that this is the Eleventh Hour.
Now you must go back and tell the people that this is The Hour.
And there are things to be considered:
This could be a good time!Where are you living?
What are you doing?
What are your relationships?
Are you in right relation?
Where is your water?
Know your garden.
It is time to speak your Truth.
Create your community. Be good to each other. And do not look outside yourself for the leader.
There is a river flowing now very fast. It is so great and swift that there are those who will be afraid. They will try to hold on to the shore. They will feel they are being torn apart, and they will suffer greatly.
Know the river has its destination. The elders say we must let go of the shore, push off into the middle of the river, keep our eyes open, and our heads above the water. See who is in there with you and celebrate.
At this time in history, we are to take nothing personally. Least of all, ourselves. For the moment that we do, our spiritual growth and journey comes to a halt.
The time of the lone wolf is over. Gather yourselves!
Banish the word struggle from your attitude and your vocabulary.
All that we do now must be done in a sacred manner and in celebration.
We are the ones we've been waiting for.
—The Elders Oraibi
Arizona Hopi Nation
See you at the end of the Helter Skelter. By the way is that me on the ride!!!
Hey Folks. Be Well. Geoff









No comments:
Post a Comment
Note: only a member of this blog may post a comment.